About OpticLine
In the United States, over 26,500 patients use peritoneal dialysis (PD) each year and are at risk for developing peritonitis, an infection of the peritoneum that causes scarring. This prevents the peritoneum from serving as an exchange membrane during PD. Currently, the only preventative measures for peritonitis are strict sanitary practices. It can take a long time to notice infection symptoms, and delayed infection detection puts patients at risk for developing peritoneal inflammation and other complications. Our team has designed a novel way to detect peritonitis before patient awareness, allowing for earlier treatment intervention. This reduces acute hospitalization costs and prevents scarring of the peritoneum, improving PD longevity. Our solution, OpticLine, uses optical methods to detect infectious levels of white blood cells (WBCs) in the dialysis waste fluid. It is inexpensive — preliminary cost analysis suggests around $50 per device when manufactured at scale — with a quick setup in under 30 seconds. OpticLine seamlessly integrates within the current PD setup, connecting in series with existing drain lines. OpticLine automatically measures and reports the patient’s WBC count so that they are immediately aware of a potential infection, reducing the time to treatment and reducing the burden on patients and their caretakers to evaluate their symptoms.
Hardware and Design
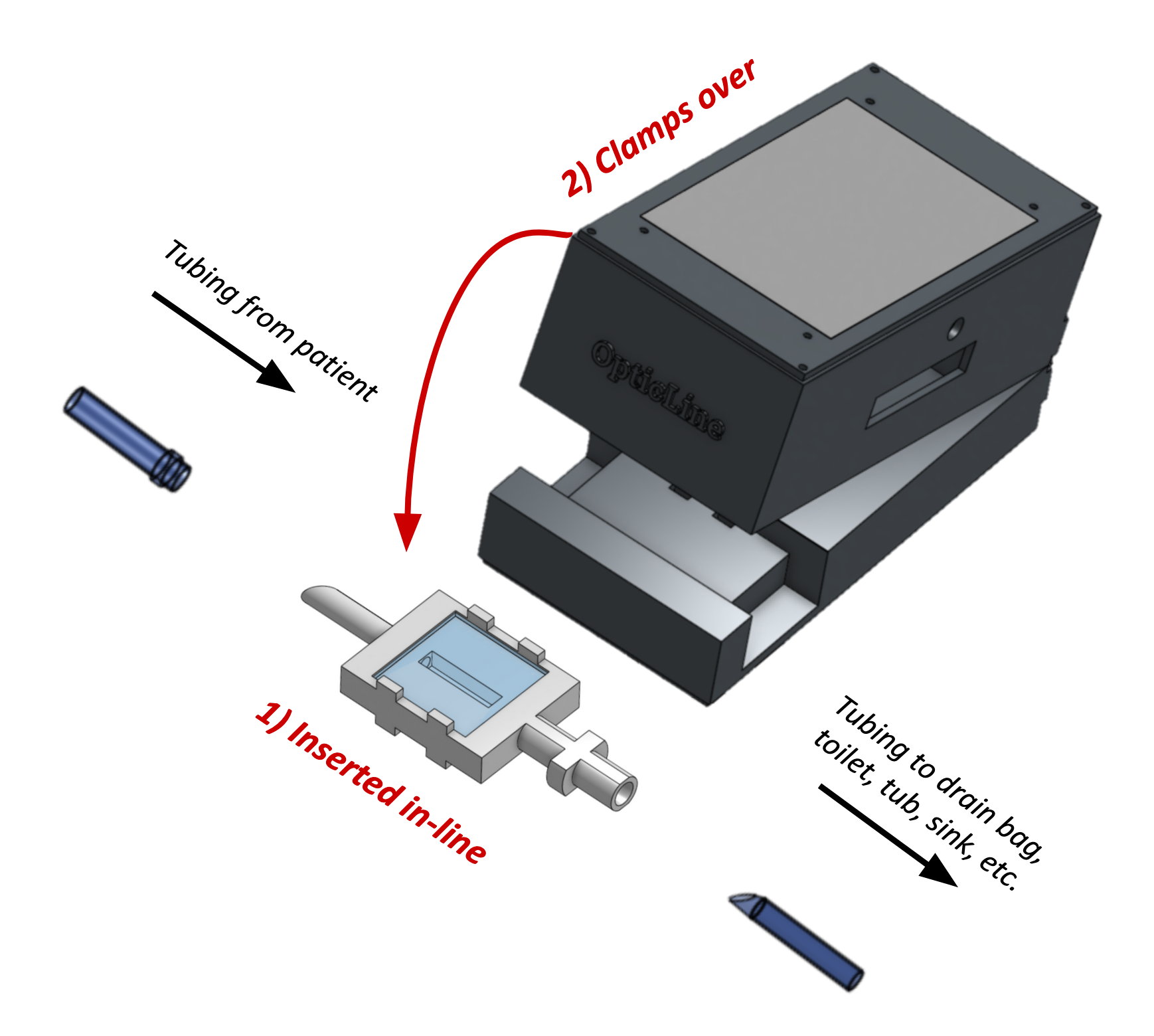
OpticLine consists of two components: a consumable viewing chamber, attached directly in-line to the PD drainage line, and the reusable clamp, which is placed over the chamber. The chamber is inserted via replicated male and female connectors to the drainage line and the drainage bag, respectively, and is replaced frequently. The OpticLine clamp houses the electronics, fastens snugly and securely over the chamber, and does not touch the effluent fluid. It is compact and powered by a standard household outlet. The clamp contains a touch screen user interface. We believe the OpticLine clamp can become even more compact once optimized for manufacturing; both items have been 3D printed for R&D but are intended to be mass-produced.
OpticLine analyzes effluent fluid in real-time through its on-board electronics and internal algorithm to estimate fluid WBC concentrations. At the end of the PD session, OpticLine then determines if the patient may be infected and if they should contact their clinical care team. It displays this information on its on-board touch screen and saves all data to its internal storage. The data can easily be accessed later from an insertable SD card or Bluetooth. We envision an easily designable app for assisting OpticLine users and displaying session information.
Testing OpticLine through R&D
Our current automated OpticLine prototype can accurately determine concentrations of white blood cells (WBCs) resuspended in real PD effluent fluid. Device measurements corresponding to healthy baseline levels (0-10 WBCs/mm3) and unhealthy levels (100 WBCs/mm3), where 100 WBCs/mm3 is clinically defined as peritonitis,1 are statistically significantly different (p < .0001).
Mean WBC concentration predictions across model testing and training iterations are displayed with ±SD, acquired after adding WBCs to sample PD effluent fluid (n=18) and running the spiked samples at 300 mL/min through the drainage tubing and into OpticLine in a lab setting replicating a PD dialysis session. The green, yellow, and red areas of the graph represent healthy, intermediate, and infected WBC concentrations, respectively. OpticLine performance compares to 1:1 correlation ("y=x" black dotted line). OpticLine has the the ability to detect fluid outflow and can thus be used for different patients, regardless of dwelling and drainage times. Our results indicate that OpticLine is able to calculate WBC concentrations in conditions representative of its intended use, suggesting with confidence that it will be a useful tool for early infection screening.
Akoh, World J Nephrol. 2012 Aug 6; 1(4): 106–122.
Human Factors & Market Research
Our team puts PD patients and their caretakers first.
In order to gather user input from key stakeholders to inform our prototyping process, we wrote an IRB protocol to administer a pilot human factors study in the form of an anonymous online questionnaire. For participants at Lucile Packard Children’s Hospital, we additionally gathered user set-up times. A summary of the pilot study results is shown on the left.